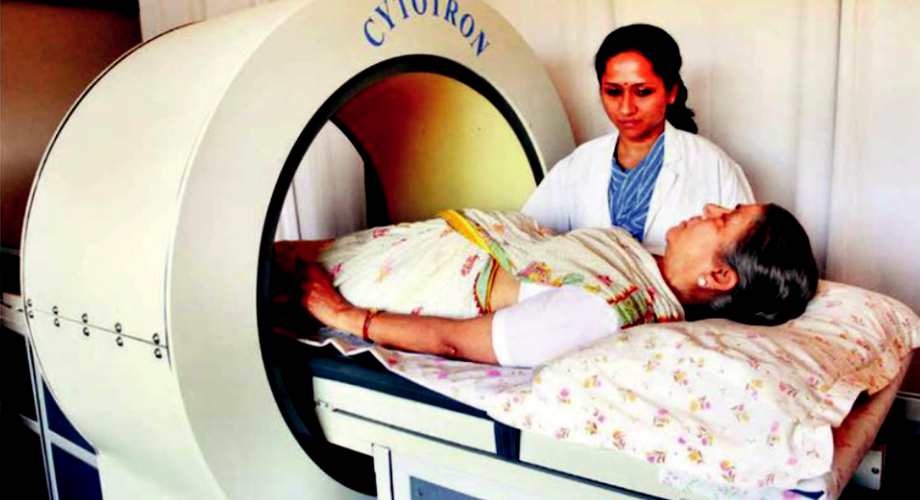
How does it work?
At first look, you might confuse the Cytotron with a whole-body MRI scanner. However, it has a much bigger bore. Inside are guns that produce high instantaneous magnetic field and radio frequencies. These frequencies are specifically targeted at the problematic tissues.
Our bodies have something called Trans-Membrane Potential pathways or TMP pathways. In case of any kind of injury, due to various reasons like disease, accident, chemical pollution, stress, and mechanical wear and tear, the body uses a mechanism to heal itself. TMP pathways play acrucial role in this healing process. Their role is to take orders to various parts of the body and aid in healing. In diseases such as cancer and arthritis, the TMP pathways suffer a breakdown. When that happens, many cellular functions get effected. This is the major cause of all non-communicable diseases.
This is where the Cytotron comes to help. The RFOMR technology that the Cytotron works on, regularizes the functions of Trans-Membrane Potential pathways, after which the repair job in various parts of the body can continue.
Is this treatment legal? Cytotron is a patented technology and is licensed only to one manufacturer ‘Scalene Cybernetics Limited, from where we have sourced the technology and setup. Cytotron is a registered trademark. The organization that manufactures it follows international standards & is certified with ISO 9001:2000, ISO 13485:2003 & other relevant standards.
On Oct. 24″ 2019, the Cytotron was granted Breakthrough Device Designation by the U.S. FDA for Breast, Liver & Pancreatic Cancers. (Link: https://www.prnewswire.com/news- releases/cyclotron-granted-breakthrough-device-designation-by- us-FDA-for-treatment-of-breast-liver-and-pancreatic-cancers- 300948903.html )
Additional solid tumor indications of use and other life-limiting indications are being pursued in parallel.
Cytotron Facts The Cytotron® has been clinically validated since 2004. European Union CE Mark-Certified for use in Musculoskeletal Disorders and Cancer (Since 2012) Awarded USA, European, and Chinese Patents US Patent – US9162076B2 European Patent – EP1753508B1 Chinese Patent – CN1960779B Off – Shore clinical validation concluded in both adult and pediatric solid tumors in Mexico City, Mexico Compassionate study in all Adult solid tumors (Brain, Breast, Colon, Prostate and Lung Cancers) at the Government Social Security Administration Hospital Compassionate study in Pediatric Brain tumors* at the National Children’s Hospital in Mexico City. *The Pediatric brain tumor trial protocol was registered with the National Institutes of Health.
(Link:https://www.clinicaltrials.gov/ct2/show/NC T03577600)
– Patient & User friendly with high patient compliance in all age groups
– Cost-effective with minimal infrastructure and maintenance costs
– Markedly reduced & affordable pricing compared to conventional or newer drugs/therapies
– More than 30 commercial and private installations worldwide