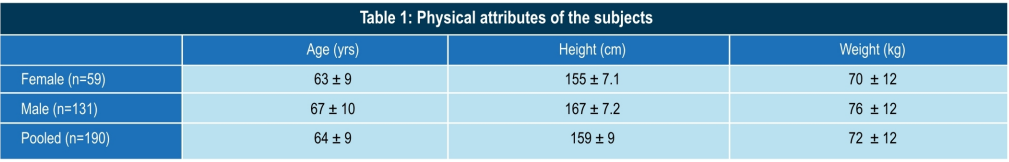
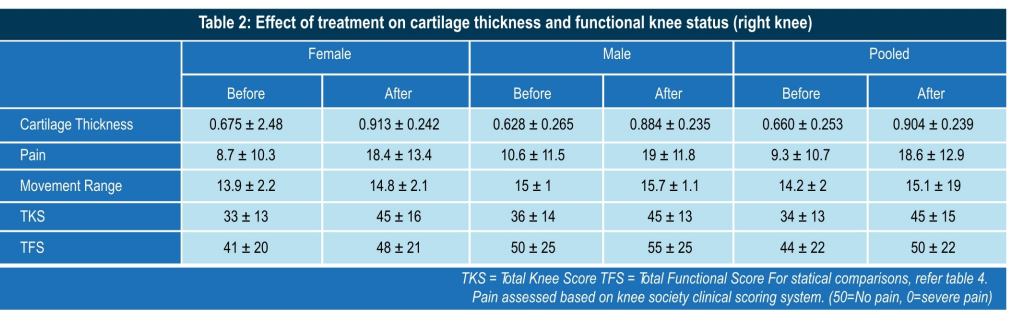
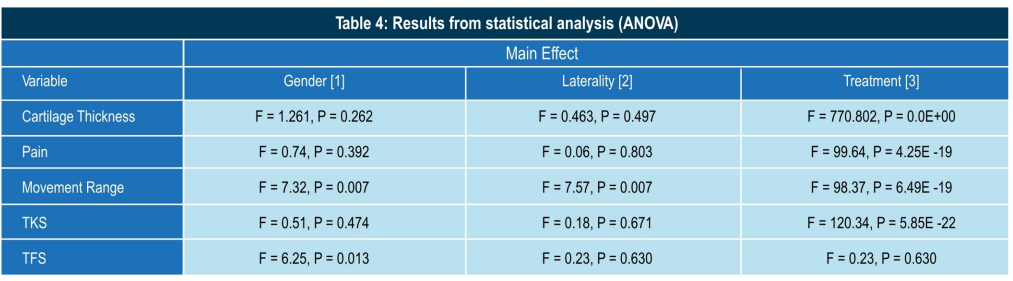
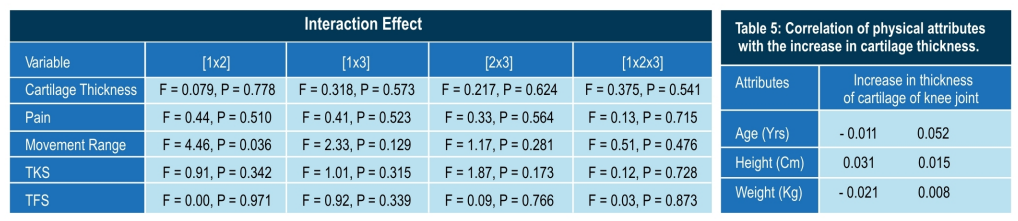
Conclusion
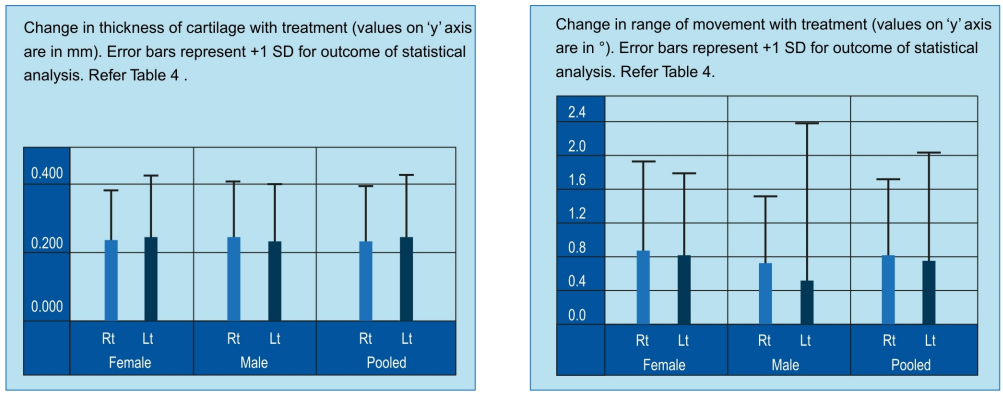
- Significant main effect, i.e. the increase in cartilage thickness, is due to the treatment only (F = 770.802;P=0.00E+00)
- There was no gender difference in the response to the treatment.
- There was no laterality effect in response to the treatment
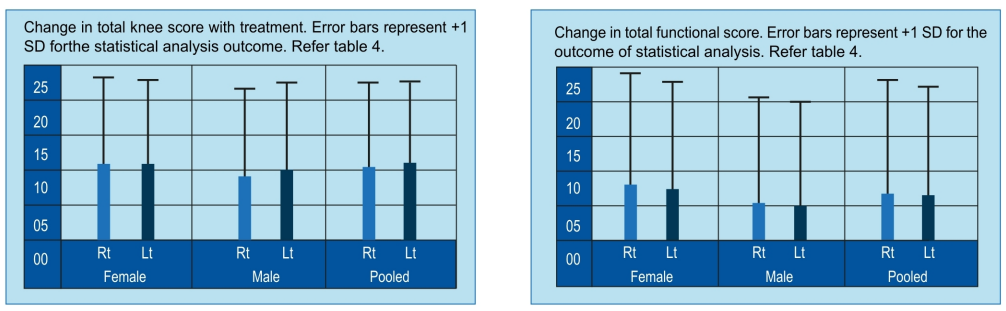
- Significant reduction in pain, increased range of movements.
- Better joint stability. Significant improvement in general quality of life.
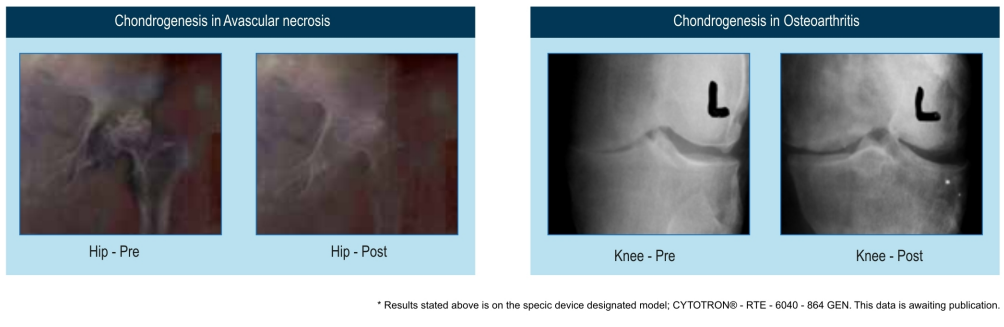
- “QMRT is the only known modality that proves cartilage re-growth- and CYTOTRON does it”
Technical Specification of CYTOTRON
Classification: Class Ila Medical Device.
Indication of use: The Device is intended to be used for Regenerative and Degenerative Tissue Engineering i.e, it can be used for regenerating tissues in-situ and to cause degeneration of uncontrolled growth of tissues.
Regeneration of tissues the current indication is for use in protein linked degenerative disorders like Osteoarthritis enabling regrowth of tissue like articular cartilage.
*Contraindications: Critically ill patients on life support system, pregnancy, patients unable to lie down for the duration of therapy, Patients with electrically,Magnetically or mechanically activated implants (eg cardiac pacemakers, Bio stimulators, Neuro stimulators, cochlear Implants, hearing aids)
MRI Incompatible implants near the target region. e.g intra medullary nail, intracranial aneurysm clips, stents, intra orbital metal fragments etc.
Patients with concomitant neoplastic disease especially Chondrosarcoma.
Drug and other device interactions: The following group of drugs that use the same or similar cellular pathways as QMRT may interact with the CYTOTRONS exposure, like Calcium Channel Blockers and Proton Pump inhibitors, NSAIDs and Alkaloids (also food containing alkaloids) for all cellular regenerative protocol. Patients should be advised to take medication with different mode of action ionizing radiations are known to damage multiplying (mitotic) cells. Hence exposure to X-ray or CT imaging of the affected joint should be avoided for at least 12 weeks after therapy. However this will not impose any risk to the patient, in case imaging is inevitable, MRI may be advised.
*Product Specifications :
The power supply Cytotron must meet these specifications:
a. Voltage fluctuation+/- 10% or less
b. Voltage imbalance +/- 3% or less
c. Frequency variation +/-4% or less
d Voltage distortion THD = 10% or less
*Recommended environmental specifications:
Operating Temperature range 18°C to 30°C, Humidity range 10% to 75% RH. Atmospheric pressure 700 to 1060 hPa, Transport/Storage Temperature range 10°C to 80°C, Humidity range 10% to 90% RH, Atmospheric pressure 500 to 1060 hPa
Mode of usage: Continuous
Life of the equipment: Typically assessed at about ten years
Typical operating voltage: 230 V, 50 Hz. single phase Maximum operating current: 12 Amps
Typical operating current: 6A
IEC classification: Type B, Class 1, IPX0
MDD classification: Class lla
Target resolution: 11.25 degrees
Power supply type: Medical grade switch mode power supply complying with EN 60601-1, EN 60601-1-2
Frequency band: VLFj LF -HF and VHF radio bands
Instantaneous magnetic field strength: 1mT to 6T for the time duration 2.0 μsec to 10 msec depends on the treatment and Dosimetry value.
E-Field: Broadband; average 100 dB V/m/MHz @ 4MHz
H-Field: Broadband; 20dB (below 1 kHz)
Broadband common mode current in control cables: 100dBmA/MHz @ 150 kHz
Fuses: Mains Fuse-12A, Isolation Transformer Fuse-2.5A
Laser Guiding System:
Output: 625 to 680 nanometer wave length less than 5 mill watt, Complies with CFR Part 1040.10 and 1040.11 Class III A LASER application
QMRT Guns:
Magnetic field
Type: MF6040-L
Guns: K- _Ferrite type; Near Field; gain; 10dB
Typical voltage: 60 Volts
Typical current:4 Amps
Radio frequency
Type of antenna:Helical
Q factor of the antenna:560
Antenna gain:13 db
Centre frequency: 68.4 MHz
3dB BW (Bandwidth): ± 4 MHz
6dB BW (Bandwidth):± 10 MHz
Input impedance:50 OHM
Input connector:SMA
Physical Dimensions:
Cytotron device dimensions:
4500mm (L) x 1210mm (W) x 1600mm (H) Weight: approx. 1900 Kg
Central control unit dimensions:
1600mm (L) x 780mm (W) x 1455mm (H) Weight: approx. 200 Kg
Coating: Powder coating/Paint and Lacquer coating body for scratch protection and prevent from corrosion
Patient weight: Max. 157 Kg
Patient bed weight: 12.95 Kg (Part No: SCL-A-01-LB)
Earthing clip weight:50 Gms (Part No: SCL-A-02-EP)
Patient transmission system: 22 Kg
(Acrylic Sheet + Metal Frame)
Total bed safe working load: 170 Kg